News
ASH 2023| TargetRx announced the results of the phase I clinical trial and preclinical study of TGRX-678
2023-12-15 Views:
Shenzhen TargetRx,Inc. (hereinafter referred to as "TargetRx"), a biotech company focus on developing precision target drug for cancer, recently unveiled the results of the Phase I clinical trial and preclinical research data for TGRX-678, a BCR-ABL tyrosine kinase inhibitor, for the treatment of patients with Chronic Myeloid Leukemia (CML). The outcomes were showcased through oral and poster presentations at the 65th Annual Meeting of the American Society of Hematology (ASH) held in San Diego, CA, the United States.

ASH 2023
December 9-12, 2023, in San Diego
Oral Presentation

Professor Jiang Qian, the principal clinical investigator of TGRX-678, gave an oral report
Title: 867 Safety and Efficacy of Tgrx-678, a Potent BCR-ABL Allosteric Inhibitor in Patients with Tyrosine Kinase Inhibitor (TKI) Resistant/Refractory Chronic Myeloid Leukemia (CML): Preliminary Results of Phase I Study Clinically Relevant Abstract Session: 632. Chronic Myeloid Leukemia: Clinical and Epidemiological: Novel Therapeutic Approaches
Session: 632. Chronic Myeloid Leukemia: Clinical and Epidemiological: Novel Therapeutic Approaches
Time: Monday, December 11, 2023: 3:15 PM
Presentation Highlights:
Background: TGRX-678 is a novel allosteric inhibitor of ABL kinases, specifically targeting the ABL Myristoyl Pocket (STAMP). In vitro data supports TGRX-678 targeting the most common BCR-ABL mutations, including T315I. Here we report an open-label, first-in-human study to evaluate the safety, preliminary efficacy, and pharmacokinetic properties of orally administered TGRX-678 in patients with resistant/refractory CML.
Methods: This study included dose escalation and expansion phases.
- During dose escalation, patients with CML in chronic or accelerated phase (CP or AP) and who had failed ≥3 prior TKIs were enrolled. Patients received orally a single dose, after a 3-5 day observation, continuously daily doses ranging from 10 to 80 mg (BID) or 40 to 240 mg (QD) were given. Dose escalation followed an adaptive Bayesian logistic regression model based on dose-limiting toxicities (DLTs) occurring in cycle 1 (28 Days).
- Dose-expansion had three cohorts:
- CML-CP without T315I mutation with ≥2 prior TKIs,
- CML-CP with T315I with ≥1 prior TKIs,
- CML-AP with ≥1 prior TKIs.
The eligible patients received continuous treatments until disease progression, intolerant toxicity, consent withdrawal, or death.
Patients: From April 30, 2021 to July11, 2023, 95 (CP n = 58, AP n = 37) patients were treated with TGRX-678 at the BID doses including 10 mg (n = 3), 20 mg (n = 6), 40 mg (n = 5), and 80 mg (n = 3), and QD doses including 40mg (n=18), 80 mg (n = 6), 160mg (n= 4) and 240mg (n=50). 40 (42%) patients were female. The median age was 45 (range 35-52) years and the median interval from diagnosis to initial TGRX-678 treatment was 97 (range 28-136) months. Median treatment duration was 8 (range 6-13) months. At baseline, 93 (98%) patients had received ≥2 lines of prior TKI therapy, among them, 78 (82%) received ≥3 lines of prior TKIs, 44 (46%) received ≥4 lines of prior TKIs. 46 (48%) patients had ≥1 ABL mutation and 28 (30%) had T315I mutation.
Efficacy results:
- Of 58 patients with CML-CP, 16 (89%) achieved complete hematologic responses (CHR), 25 (43%) major cytogenetic responses (MCyR), 19 (33%) complete cytogenetic responses (CCyR), and 11 (19%) major molecular responses (MMR).
- Of 14 CML-CP patients with T315I mutation, 9 (100%) achieved CHR, 9 (62%) MCyR, 8 (57%) CCyR and 7 (50%) MMR.
- Of 35 CML-CP patients without any ABL mutations, 12 (100%) achieved CHR, 11 (31%) MCyR, 6 (17%) CCyR and 1 (3%) MMR (Fig. A).
- Of 9 CML-CP patients with other ABL mutations, 4 (67%) achieved CHR, 5 (56%) MCyR, 5 (56%) CCyR and 3 (33%) MMR (Fig. B).
- Of 37 CML-AP patients, 31 (84%) had major hematologic response, 13 (35%) MCyR, and 9 (24%) CCyR (Fig. C).
- There were 22 CML-CP and 23 CML-AP patients previous received 3rd Gen TKIs (Ponatinib or HQP1351) or Asciminib. Of 22 CP patients, 21 (95%) achieved CHR, 4 (18%) MCyR and 3 (14%) CCyR. Of 23 CML-AP patients, 17 (74%) achieved CHR, 5 (22%) MCyR and 3 (13%) CCyR.
Pharmacokinetics (PK): The PK results suggested that TGRX-678 exposure (Cmax and AUCtau) was dose-proportional within range of 10mg~80mg BID or 40mg~240mg QD. The elimination of TGRX-678 in human plasma is slow, and the half-life (T1/2) of which is about 120 hours. At steady state, the accumulation ratios of Cmax and AUCtau are 3.7-8.0 and 5.4-11.8, respectively. The high accumulation ratio of TGRX-678 resulted in not only an increase of plasma exposure, but also a decrease of the gap between Cmax and Ctrough.
Safety results: 7 DLTs were observed, one case of elevated alanine aminotransferase at 20 mg BID and five cases of thrombocytopenia at 40mg QD (1), 40mg BID (2) and 80mg QD (2) and one hepatic function abnormal at 240 mg QD. However, MTD was not reached according to protocol criteria. Most treatment-related adverse events (TRAEs) were grade 1-2. AEs ≥ grade 3 that happened more than 5% were thrombocytopenia (50%), neutropenia (42%), anemia (24%) and hypertriglyceridemia (9%). In total 15 patients discontinued the study due to disease progression (5, all APs), intolerance (2), physician’s decision (2), consent withdrawal (6). There was one death occurred which was not drug related.
Conclusions: Clinical activity of TGRX-678 was seen in all cohorts and across TKI-resistant mutations including T315I, providing a promising treatment option for CML CP/AP patients, including those who failed Ponatinib or Asciminib. Its unique PK properties might bring additional benefit to patients. TGRX-678 was well tolerated in heavily pretreated CML patients. Currently two doses were selected to optimize the RP2D.
Study Identifier: NCT05434312.
Poster Presentation
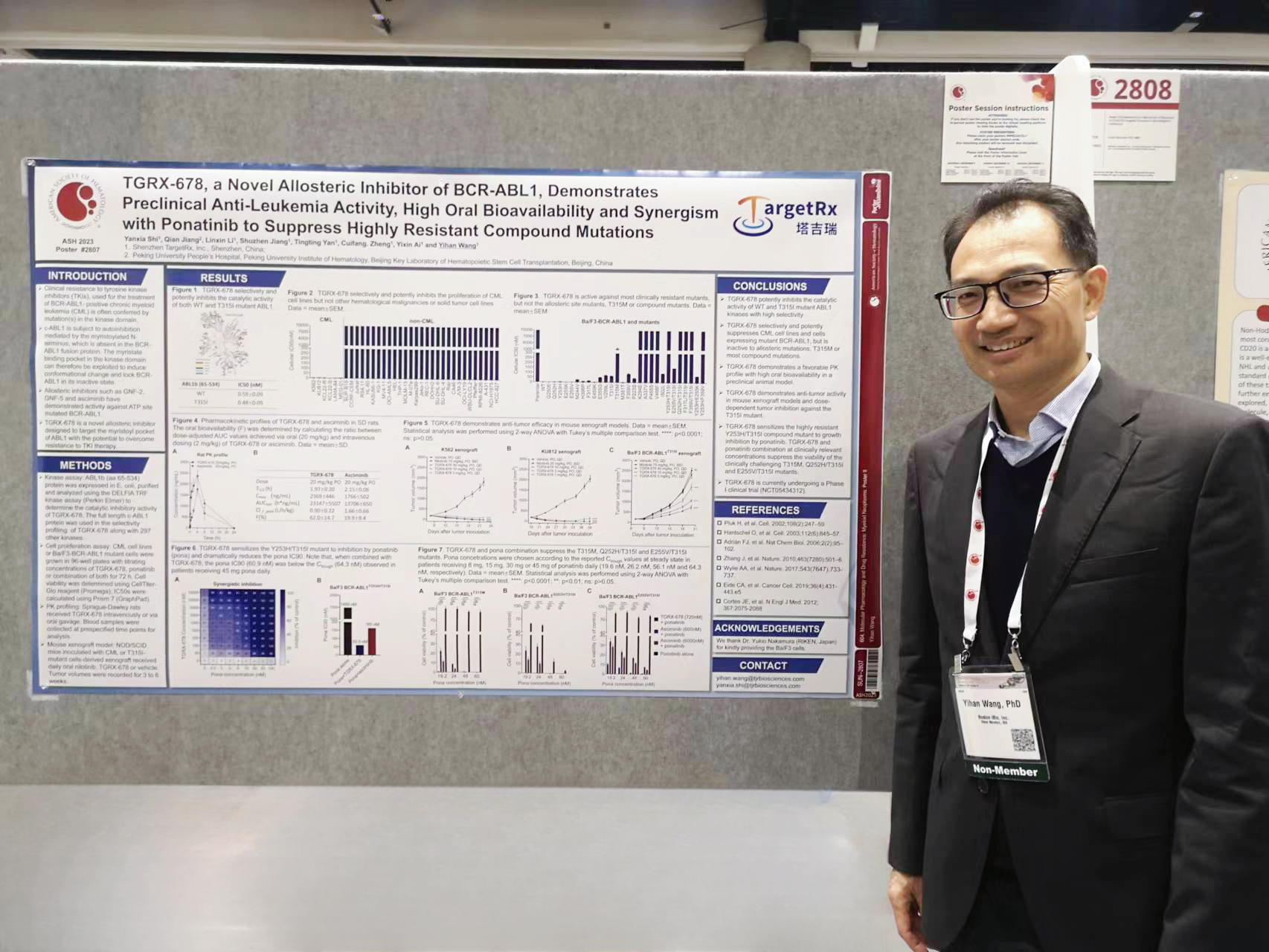
Dr. Wang Yihan, Chairman of TargetRx, gave a poster presentation at the scene
Title: TGRX-678, a Novel Allosteric Inhibitor of BCR-ABL1, Demonstrates Preclinical Anti-Leukemia Activity, High Oral Bioavailability and Synergism with Ponatinib to Suppress Highly Resistant Compound Mutations
Session: 604. Molecular Pharmacology and Drug Resistance: Myeloid Neoplasms: Poster II
Time: Sunday, December 10, 2023, 6:00 PM-8:00 PM
Presentation Highlights:
Introduction: Clinical resistance to the treatment of BCR-ABL1-positive chronic myeloid leukemia (CML) patients is often conferred by mutation(s) in the ATP-binding site of ABL1 kinase domain. ABL1 is subject to auto-inhibition mediated by myristoylation-triggered conformational change, which can be exploited to overcome resistance, as validated by allosteric inhibitors such as GNF2, GNF5 and Asciminib. TGRX-678, a novel allosteric inhibitor designed to target the myristoyl pocket of ABL1, is currently being investigated in a phase I clinical trial (NCT05434312). Herein we report the selectivity, potency, anti-leukemia activity and in vivo pharmacokinetic (PK)/pharmacodynamic(PD) characteristics of TGRX-678.
Methods: For in vitro study, ABL1b 65-534 fragments were used following the protocol for Z'-LYTE kinase assay kit (Thermo Fisher). For cellular assays, CML cell lines or engineered Ba/F3 cells were incubated with titrating TGRX-678 for 72 hrs, then CellTiter Glo reagent (Promega) was added and the chemiluminescence was measured. For in vivo studies, SD rats received TGRX-678 or Asciminib and plasma samples were collected for analysis. NOD-SCID mice were inoculated with CML or Ba/F3-BCR-ABL1T315I cells and received oral TGRX-678. Tumor volumes and body weights were recorded for 3 to 5 weeks.
Results:
TGRX-678 exhibited minimum off-target inhibition at 1 μM in a panel of 300 kinases, but inhibited the enzymatic activity of both ABL1 and the T315I mutant with IC50 values of 0.59 nM and 0.48 nM, respectively.
The anti-proliferative activity of TGRX-678 was determined in CML cell lines including K562, KU812, KCL22-S, KCL22-R, LAMA-84 and MEG-01 with IC50 values ranging from 1.1 nM to 6.56 nM. TGRX-678 had no cytotoxicity in 29 cell lines representing other hematological malignancies and solid tumors. Using engineered Ba/F3 cell lines, TGRX-678 was active against the native BCR-ABL1 (IC50 = 4.11 nM) and the clinically prominent mutants including G250E (IC50 = 2.49 nM), Q252H (IC50 = 2.38 nM), Y253H (IC50 = 5.6 nM), E255K (IC50 = 1.01 nM), E255V (IC50 = 2.73 nM) and T315I (IC50 = 66.1 nM). The cellular activity of TGRX-678 was attenuated by mutations in the myristoyl pocket such as A337V, P465S, V468F and I502L or mutations in the SH3-kinase domain interface including P223S and K294E, underpinning its allosteric mode of action.
In SD rats, oral administration of TGRX-678 at 20 mg/kg achieved a maximum plasma concentration (Cmax) of 2369 ng/mL, which was 1.3 times that of Asciminib. The overall exposure (AUClast) of TGRX-678 (23,147 h·ng/mL) was also 1.7 times that of Asciminib. The oral bioavailability of TGRX-678 was 62%. In xenograft-bearing mice, oral dosing of TGRX-678 at 1 mg/kg QD (for KU812) or 3 mg/kg QD (for K562) was sufficient to completely inhibit tumor growth. In the Ba/F3-BCR-ABL1T315I xenograft mice, TGRX-678 induced dose-dependent tumor regression and reduced tumor volume by 67% at 45 mg/kg QD dose.
We further examined the synergistic effect of TGRX-678 and ponatinib combination. While each agent was inactive to the Y253H/T315I mutant, the combination of TGRX-678 and ponatinib inhibited cell proliferation at concentrations within accessble clinical dose levels. The addition of TGRX-678 dramatically lowered the IC90 for ponatinib from 1489 nM to 60.9 nM, whereas asciminib only reduced the IC90 to 165 nM.
In addition to the Y253H/T315I compound mutation, using E255V/T315I, Q252H/T315I and T315M engineered mutant cells to test single-agent Ponatinib/TGRX-678/Asciminib, and compared the combined inhibitory activity of point concentrations near steady-state plasma concentrations at different clinical doses of Ponatinib. The test results also demonstrated the activity advantages of Ponatinib combined with TGRX-678 compared with Asciminib.
Conclusion: Our findings support that TGRX-678, a novel allosteric inhibitor of BCR-ABL1, is potent against the CML cells and cell lines harboring clinical ATP-site mutations with minimum non-specific cytotoxicity. TGRX-678 exhibits a favorable PK profile and anti-tumor activity in vivo. Furthermore, the strong synergistic effect of TGRX-678 and Ponatinib in T315M or T315I-inclusive compound mutations provides new possibilities to overcome current clinical drug resistance challenges This data warrant further clinical investigation of TGRX-678 for the treatment of resistant or refractory CML patients.
About ASH
As one of the largest and most authoritative hematology academic events in the world, the American Society of Hematology Annual Meeting (ASH) brings together well-known hematology experts from various countries every year to share the world's most cutting-edge research progress and breakthrough clinical research data.
About TGRX-678
TGRX-678 is a class of drug for the treatment of chronic myelogenous leukemia (CML) independently developed by TargetRx, with global independent intellectual property rights and a brand-new drug structure. Different from the approved 1st to 3rd generation targeted drugs that act on the catalytic site, TGRX-678 targets the allosteric site (STAMP) of the BCR-ABL fusion gene and has the ability to against resistance to existing marketed drugs. It has the potential to provide a promising treatment option for patients with CML who have failed Ponatinib and Asciminib. TGRX-678 is currently undergoing clinical studies in China and the United States (NCT05434312).
About TargetRx
TargetRx designs, screens, evaluates, optimizes and develops a series of original and latest generation small molecule targeted anti-tumor drugs with independent intellectual property rights, and is committed to challenging the upgraded version of cancer - the medical problem of acquired drug resistance. Since its establishment, it has applied for more than 300 patents in China, the United States, Europe, Japan, etc.
Currently, TargetRx's two main products - the latest generation of novel mechanism drug TGRX-678 for the treatment of chronic myelogenous leukemia (CML) and the internationally leading third-generation ALK inhibitor TGRX-326 for the treatment of non-small cell lung cancer (NSCLC) are being developed. Multi-center clinical studies are carried out across China. TargetRx's other innovative targeted drug product lines are also being actively promoted.
Prev: TargetRx Signs Collaboration Agreement With Simcere Zaiming
Next: ASH 2023 | TargetRx to highlight advances of TGRX-678 in CML






 Tel: +86-0755-86934300
Tel: +86-0755-86934300 3rd Floor, Building A1, Kexing Science Park, No. 15 Keyuan Rd., Nanshan District, Shenzhen, China
3rd Floor, Building A1, Kexing Science Park, No. 15 Keyuan Rd., Nanshan District, Shenzhen, China E-mail:
E-mail: 