News
TargetRx announces dosing of first subject in first-in-human phase I clinical trial of TGRX-678, an allosteric BCR-ABL inhibitor with novel mechanism against chronic myelogenous leukemia
2021-06-24 Views:
Recently, Shenzhen TargetRx Inc. (hereinafter referred to as TargetRx), a leader in small molecule targeted drugs, announced that the first subject has been dosed in the first-in-human clinical trial of its allosteric Bcr-Abl inhibitor TGRX-678 with a new mechanism against Chronic Myelocytic Leukemia (Chronic Myelocytic Leukemia, CML) at Peking University People's Hospital.
CML is a malignant tumor caused by the clonal proliferation of bone marrow hematopoietic stem cells. In affected cell lines, Philadelphia (Ph) chromosome and/or BCR-ABL fusion gene[1] can be identified. The annual incidence of CML in China is 0.39/100,000 to 0.99/100,000. The survey results show that the median age of onset of CML patients in China is 45-50 years old, which is younger than the median age (67 years old) of onset of CML in Western countries[2].
BCR-ABL inhibitors currently used in clinical treatment of CML include the first-generation inhibitorimatinib, the second-generation inhibitors nilotinib, dasatinib, bosutinib and flumatinib, the third-generation inhibitor ponatinib (not yet marketed in China), etc. Due to the slow onset of CML, there are often no symptoms in the early stage. Some patients are already in the late stage at the time of diagnosis, and the late-stage patients have poor tolerance to the drugs, low remission rate and very short remission period[1]. The hit movie Dying to Survive in 2018 has aroused the attention of the public to CML and the dilemma of no drugs available for the CML patients because domestic CML patients may not be able to afford the expensive imported innovator drugs, or are resistant or intolerant to existing drugs.
TGRX-678 is a first-in-class anti-tumor drug independently developed by TargetRx, with independent intellectual property rights and a novel drug structure. Different from the first- to the third-generation targeted drugs that act on the catalytic site, TGRX-678 targets the allosteric site of the BCR-ABL fusion gene and can be used to treat CML patients with resistance or intolerance to existing drugs (including resistance by the T315I gatekeeper residue mutation).
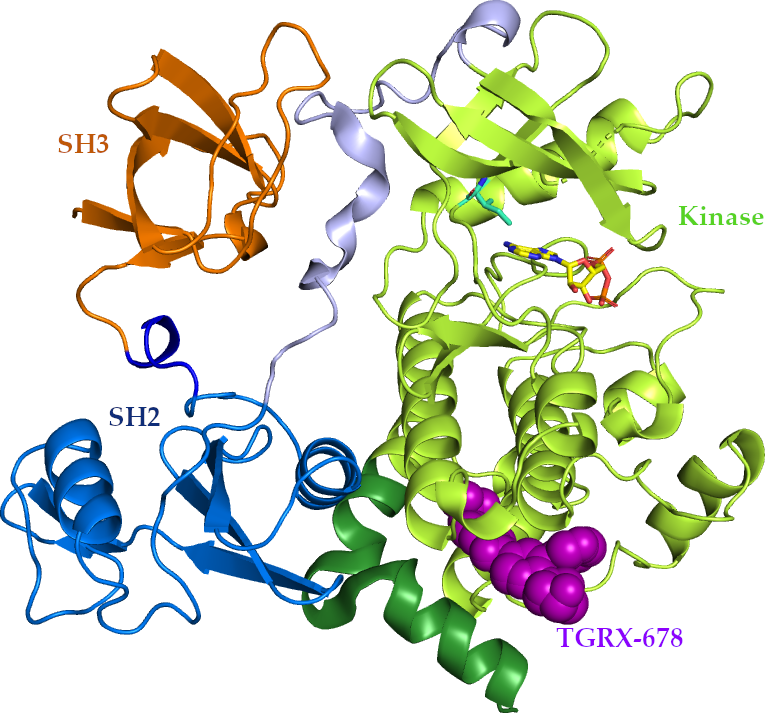
The main purpose of the phase I clinical trial conducted at Peking University People's Hospital is to explore the safety and tolerability of multiple doses of TGRX-678 tablets in humans, and to determine the maximum tolerated dose (MTD) and the recommended phase 2 dose (RP2D).
Dr. Yihan Wang, the founder and CEO of TargetRx, has decades of overseas experience in the R&D of targeted anti-cancer small molecule drugs, and very rich experience in the field of drug design. At the same time, he is the core inventor of the third-generation CML first-in-class drug IClusig® (ponatinib) which is the only one approved by the FDA in the world.
Regarding the successful completion of the first-in-human (FIH) administration of TGRX-678, Dr. Wang said, in the structural design of TGRX-678, we have adopted a completely different site and mechanism of action from the previous three generations of drugs, and it has an excellent selectivity. TGRX-678, as the first Bcr-Abl inhibitor with a new mechanism of action to enter clinical trials in China, and is expected to elevate the treatment of leukemia in China to the international advanced level. We eagerly hope to obtain an exciting clinical trial result, so that CML patients can use internationally leading and affordable domestic new drugs, improve the quality of life of patients, and guarantee their lives. Currently, the patient recruitment for clinical trial of TGRX-678 is actively underway.
---------------------------------------------References:
[1] Ge Junbo, Xu Yongjian, Wang Chen. Internal Medicine. Ninth Edition. Beijing: People's Medical Publishing House, 2018
[2] Guidelines for Diagnosis and Treatment of Chronic Myelogeous Leukemia in China (2020 Edition). Chin J Hematol, May 2020, Vol. 41, No. 5.






 Tel: +86-0755-86934300
Tel: +86-0755-86934300 3rd Floor, Building A1, Kexing Science Park, No. 15 Keyuan Rd., Nanshan District, Shenzhen, China
3rd Floor, Building A1, Kexing Science Park, No. 15 Keyuan Rd., Nanshan District, Shenzhen, China E-mail:
E-mail: 