News
TargetRx's next-generation Bcr-Abl inhibitor used to treat CML has been cleared for clinical trials in China
2020-06-04 Views:
On June 3, 2020, the fourth-generation Bcr-Abl inhibitor TGRX-678 independently developed by Shenzhen TargetRx Inc. was officially cleared for clinical trials via the notification issued by the Center for Drug Evaluation (CDE) of the National Medical Products Administration. The clinical application for TGRX-678 only lasted 37 working days from its acceptance to approval.
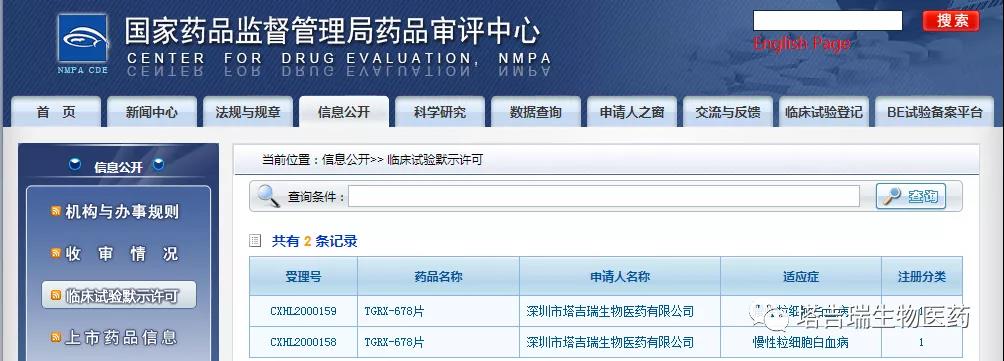
Source: official website of the Center for Drug Evaluation of the National Medical Products Administration
TGRX-678 is a fourth-generation tyrosine kinase inhibitor that targets the Bcr-Abl fusion gene. It is independently developed by TargetRx. Its mechanism of action is completely different from all other Bcr-Abl inhibitors currently on the market and it can be used for treatment of CML patients that are resistant or intolerant to the first, second, and third-generations inhibitors. TargetRx is accelerating the progress of the clinical trial of TGRX-678, striving to bring treatments that are more effective to patients with CML as soon as possible.
At the same time, TargetRx's other innovative targeted drug product lines are also actively advancing. Since its establishment five years ago, TargetRx has been deeply involved in the field of targeted anti-cancer drugs. By striving to address the most challenging resistance problems of targeted anti-cancer drugs in clinical practice worldwide, TargetRx has achieved fruitful results through innovation, and thus been recognized by all sectors of society. TargetRx will continue to maintain its enthusiasm for scientific research and bring a wider range of cutting-edge innovative therapies to patients.






 Tel: +86-0755-86934300
Tel: +86-0755-86934300 3rd Floor, Building A1, Kexing Science Park, No. 15 Keyuan Rd., Nanshan District, Shenzhen, China
3rd Floor, Building A1, Kexing Science Park, No. 15 Keyuan Rd., Nanshan District, Shenzhen, China E-mail:
E-mail: 