News
CDE has accepted the clinical application of TargetRx's fourth-generation Bcr-Abl inhibitor
2020-04-13 Views:
On April 11, 2020, Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) officially accepted the clinical trial application of TGRX-678, a fourth-generation Bcr-Abl inhibitor independently developed by Shenzhen TargetRx Inc., which is mainly used for the treatment of chronic myeloid leukemia. Pre-clinical studies have shown that TGRX-678 has high activity against existing drug resistance mutations in clinical practice, especially the T315I gatekeeper residue mutation.
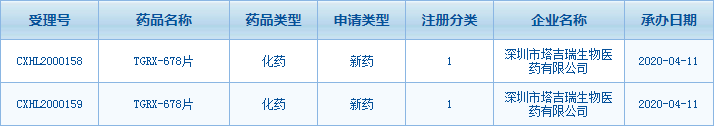
Compared with the previous three generations of Bcr-Abl inhibitors, the fourth generation adopts a new mechanism of action, has excellent selectivity, and can act well on existing resistance mutations. Moreover, pre-clinical animal experiments show that its toxic and side effects are extremely low, and the predicted treatment window is very considerable.

The heat broadcast of the movie Dying to Survive in 2018 brought the current situation of chronic myelogenous leukemia and cancer patients' difficulties in the access to medication into the public's field of vision. What is not mentioned in the movie is that the “magic drug” will become ineffective after being taken for a period of time due to the patient's drug resistance. The patient needs to constantly substitute the next-generation drugs in order to survive for a longer time. The only third-generation drug approved in the world, Ponatinib, was developed by Dr. Yihan Wang, the founder and chairman of TargetRx, and his colleagues at ARIAD Pharmaceutical Inc. (USA) for more than ten years. Unfortunately, although Ponatinib was approved for marketing by FDA as early as 2012, it has not entered China so far. After the screening of the movie, many media interviewed Dr. Wang. In these interviews, Dr. Wang told his mission of saving the lives of cancer patients and his determination to lead TargetRx to develop the world's leading innovative Chinese local medicine.
For details, please see"Yihan Wang: I am not a god of medicine, but just a dream chaser on the road of scientific research"、"Yihan Wang: "I am not a god of medicine, I just want to give them hope"、"Yihan Wang from TargetRx: Behind Gleevec, a next-generation anti-cancer silver bullet emerges"
TargetRx is a biopharmaceutical company focusing on the R&D of next-generation small molecule targeted anticancer drugs, and it is committed to addressing the major medical problems of acquired cancer drug resistance. Through its core technologies, TargetRx designs and synthesizes highly specific anti-drug-resistant active molecules. Since its establishment, TargetRx has applied for more than 100 patents. In addition to TGRX-678, TargetRx's main products under development include the third-generation product for ALK-positive non-small cell lung cancer, and its clinical trial application will be submitted in the near future.
Top talents from anywhere wanted here. We sincerely welcome more talents with a common vision to join our team, work together with us to tackle key problems and strive to bring revolutionary and innovative therapies to patients as soon as possible. (For details, please see: In 2020, TargetRx is waiting for you to join).






 Tel: +86-0755-86934300
Tel: +86-0755-86934300 3rd Floor, Building A1, Kexing Science Park, No. 15 Keyuan Rd., Nanshan District, Shenzhen, China
3rd Floor, Building A1, Kexing Science Park, No. 15 Keyuan Rd., Nanshan District, Shenzhen, China E-mail:
E-mail: 